The gypsy moth caterpillar is a serious defoliator of broadleaved forests in eastern North America. In addition, this pest defoliates trees and shrubs in residential areas causing economic and aesthetic impacts and, when infestations are heavy, creates a nuisance to residents. Caterpillars prefer hardwoods, but may feed on several hundred different species of trees and shrubs. During periods when gypsy moth populations are dense, larvae feed on almost all vegetation. Trees weakened by consecutive defoliation are vulnerable to attack by disease organisms and other insects. For example, the Armillaria fungus attacks the roots of weakened trees, and the two-lined chestnut borer attacks the trunk and branches.
Life Cycle
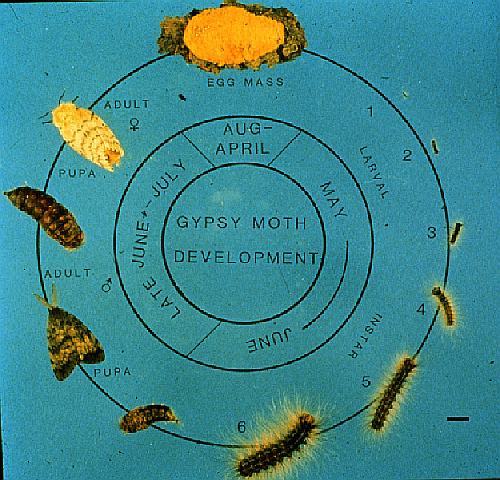
The gypsy moth has one generation per year passing through four
life stages: egg, caterpillar, pupa, and adult (moth stage) (Figure
1). Only the caterpillar which reach maturity between mid-June
and early July defoliate trees and shrubs. After 6 to 8 weeks,
the caterpillar enters the pupal stage for 7 to 14 days, which
changes into adults (moths). Flightless female moths mate and
lay their eggs in masses in July and August. Four to six weeks
later, embryos develop into caterpillars. The caterpillars remain
in the eggs during the winter. The caterpillars emerge from the
eggs the following spring, coinciding with budding of most broadleaved
trees (McManus et al 1989).
Distribution
The gypsy moth is not native to North America but was introduced
from Europe in 1869 near Boston, Massachusetts. Historically,
populations of the gypsy moth have undergone periodic outbreaks
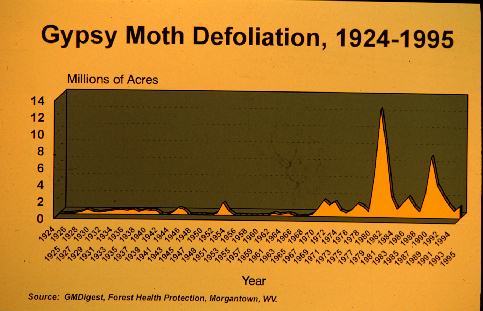
to extremely high densities that resulted in widespread defoliation
to an average of 3.0 million forested acres per year. More recently
(1992 through 1996), populations have been declining to an average
of 1.0 million forested acres per year partly due to the rapid
spread of an introduced fungus (Figure 2).
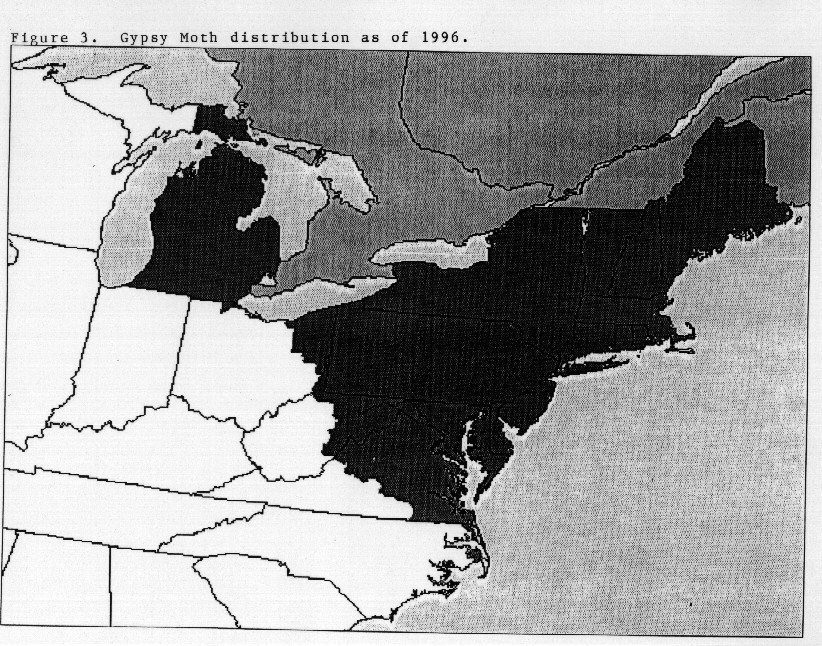
Since the introduction of the European or North American strain
of gypsy moth, it has spread south and west, and continues to
spread along the leading edge of infestation at the rate of approximately
12 miles per year (Figure 3). The Asian strain of gypsy moth
was recently introduced on the East and West coasts of North
America and eradicated.
Biological Control of the Gypsy Moth
Pathogens -- In eastern North America, the gypsy moth is subject
to a variety of naturally occurring infectious diseases caused
by several kinds of pathogens -- bacteria, fungi, and a nucleopolyhedrosis
virus (NPV). The NPV, which was inadvertently introduced with
the gypsy moth or its parasites, and an introduced fungus Entomophaga
maimaiga cause widespread mortality and are described here.
The other pathogens cause only limited mortality.
Virus
The naturally occurring disease caused by the NPV is often referred
to as "wilt" due to the soft, limp appearance of the
diseased larvae (Figure 4). The disease can reach outbreak (epizootic)
proportions as gypsy moth population densities increase. These
outbreaks result from increased transmission rates, within and
between generations of the gypsy moth, as small caterpillars become
infected and die on leaves in the crowns of trees. These caterpillar
cadavers disintegrate and serve as inocula for healthy feeding
caterpillars. Also, virus transmission occurs when adult females
deposit their egg masses on NPV-contaminated surfaces. Caterpillars
hatching from these contaminated eggs in the following spring
have a high risk of contracting the disease. Birds and mammals
have the ability to pass and disperse active gypsy moth NPV, and
parasites and invertebrate predators may play a role in the transmission
of gypsy moth NPV within natural populations. In many dense gypsy
moth populations, the virus kills up to 95% of the larvae and
reduces populations to levels where they cause only minimal defoliation
and tree damage in the following year (Reardon and Podgwaite 1992).
In the late 1950's, the USDA Forest Service began to explore the
feasibility of developing this pathogen as an alternative to chemical
insecticides for suppressing gypsy moth populations. In April
1978, the gypsy moth nucleopolyhedrosis virus product Gypchek
was registered for use by the Environmental Protection Agency
(US EPA). Today, Gypchek is produced in live gypsy moth caterpillars
in the laboratory by the USDA Forest Service and Animal and Plant
Health Inspection Service (APHIS), processed, and made available
for aerial and ground application as part of the Federal and State
Cooperative Suppression Program.
Fungus
In 1908, pest managers in the Boston area introduced the fungus
Entomophaga maimaiga via infected gypsy moth larvae
collected in Japan. Releases continued until 1911, when the local
gypsy moth populations collapsed and there was no way to continue
propagating the fungus. In June and July 1989, E. maimaiga
was first recovered in North American gypsy moth and was found
causing extensive epizootics in populations of gypsy moth in seven
contiguous northeastern States (Connecticut, Massachusetts, Vermont,
New Hampshire, New Jersey, New York and Pennsylvania). By 1990,
the fungus was also recovered in three more northeastern states
(Maine, Delaware, Maryland) and in southern Ontario. Today E.
maimaiga occurs in most areas where the gypsy moth occurs
and is prevalent in low-to-high density gypsy moth populations,
causing up to 95% mortality of large caterpillar (Figure 5).
The fungus is highly variable, and as yet unpredictable, in reducing
gypsy moth populations. It is not applied as a direct control.
Fungal resting spores in soil and infected gypsy moth cadavers
are collected and dispersed by hand to spread the fungus to new
locations although natural spread has been fairly rapid (Reardon
and Hajek 1993).
Parasites -- Using parasites against the gypsy moth has been one
of the most massive programs in biological control history (Reardon
1981).
1905 to 1980
From 1905 to 1980, approximately 78 species of parasites (over
200,000 individuals) were sent to the USDA Agricultural Research
Service (ARS) quarantine facilities in the United States. Of
these, approximately 53 species were shipped to cooperating agencies
for initiation of laboratory colonies or release. Between 1905
and 1914, gypsy moth caterpillars and pupae containing parasites
were collected in Europe, Japan, and Russia and shipped to the
United States. Six of the parasite species imported and introduced
became established (Table 1). Parasite importation was reinstated
in 1922 to 1933 with searching for gypsy moth infestations in
France, Spain, Italy, Germany and Japan. These efforts led to
the establishment of two flies and the possible establishment
of one wasp (Table 1).
During both periods of foreign exploration, 1905-1914 and 1922-1933,
hosts and parasites were collected from high-density gypsy moth
populations. Limited foreign exploration was resumed in the 1960's,
and in the 1970's ARS established gypsy moth projects at their
European Parasite Laboratory in France and Asian Parasite Laboratory
in Japan. Only one exotic species of parasite was established
during this period probably due to numerous problems associated
with rearing and releasing parasites. Problems with rearing include
inadequate taxonomic identification and poor and variable host
quality and quantity. Problems with releasing parasites include
inadequate numbers, "laboratory" strains that were not
adaptable to field conditions, lack of alternate or overwintering
hosts, and lack of host density and habitat requirements. One
exotic species of parasite was released in the late 1960's and
1970's but was not recovered until 1996. Several parasites native
to the United States have became opportunistic parasites of the
gypsy moth, that is, when gypsy moths are available. The augmentation
approach either as inundative releases (released individuals regulate)
or inoculative releases (progeny of released parasites regulate
generations of the gypsy moth) has been attempted with numerous
species against artificial and natural gypsy moth populations.
In general the incidence of parasitism by the released species
increased, but no impact on gypsy moth populations was detected.
Also, combinations of natural enemies (e.g. aerial application
of the bacterial insecticide Bt and releases of Cotesia
melanoscela, to transmit NPV) have been used with limited
success.
1980 to 1992
Prospects for using classical, and augmentation approaches to
improve biological control of the gypsy moth were explored again
during the 1980's and early 1990's. Foreign exploration for parasites
shifted to Asia, and 17 parasite species were received at ARS
quarantine in the United States. Most of these were from Korea,
Japan, and India (parasites of Indian gypsy moth, Lymantria
obfuscata Walker), whereas little material was obtained
from the other promising regions, China and the Russian Far East.
Releases of 15 species were made, but establishment of only one
species, the pupal parasite Coccygomimus disparis
(Viereck) was confirmed. This species appears to be dispersing
well over the generally infested area, but with limited effectiveness
against the gypsy moth because it parasitizes numerous species.
1993 to 1997
Recent interest in the classical approach to biological control
has been provided through the National Biological Control Institute
(USDA APHIS) and "New Directions in Biological Control of
the Gypsy Moth" with efforts focusing in non-outbreak sites
on promising species that have not been previously introduced.
Dominant species from southern Europe that failed to become established
in New England or the Middle Atlantic States (e.g., Glyptapanteles
porthetriae (Muesebeck)) are being imported and reared
for release in the southern states with different forest habitat
types, climate, and availability of alternate host species (Fuester
1993). Manipulative experiments conducted in New England suggest
that artificial elevation of gypsy moth populations might be useful
for maintaining populations of insects that parasitize caterpillars,
such as Compsilura concinnata (Meigen), Parasetigena
silvestris (Robineau-Desvoidy), and Cotesia melanoscela
(Ratzeburg).
Predators -- Many species of animals in the United States eat
the gypsy moth and other defoliating insects. The gypsy moth
predator community is complex and includes about 50 species of
birds and 20 species of mammals, along with some amphibians, reptiles,
fish, insects, and spiders. Only a few of these predators are
known to affect gypsy moth population dynamics. The predators
are all opportunistic feeders, which means that their taste for
the gypsy moth depends upon the scarcity of other preferred foods.
Vertebrate predators, especially the white-footed mouse (Peromyscus
leucopus), are major sources of large caterpillar and pupal
mortality in low density gypsy moth populations. Recent studies
of bird predation tend to show that gypsy moth is not a major
food item of most species.
Insect predators especially ants and the imported carabid beetle
Calosoma sycophanta (Figure 6) have a limited impact
on gypsy moth populations. Calosoma sycophanta
was imported from Europe in 1905-1910 and became established easily.
It is common throughout most of New England and extends into
New York, New Jersey, central Pennsylvania, and northeast Maryland.
The beetle is a specific predator of gypsy moth and usually associated
with high density gypsy moth populations.
Conclusions
In general, parasites together with other natural enemies (predators
and pathogens) help regulate populations of the gypsy moth by
reducing their numbers. Whether these introduced parasites have
reduced the average population density of the pest or lengthened
the period between outbreaks is difficult to determine. The rate
of parasitism from a particular parasite species varies from site
to site and from year to year, depending on such factors as the
number of gypsy moth larvae, the number of alternate hosts, and
the weather. Eleven exotic species of parasites have been established
and continue to disperse along with the gypsy moth. Natural enemies
are thought to help maintain low density populations, but not
to prevent the buildup of already increasing populations. Foreign
exploration for natural enemies has occurred throughout most of
the native range of the gypsy moth. In the continued search for
biological control agents, selection of candidates focuses on
species that are (1) from low density gypsy moth populations,
(2) limited to one generation per year, (3) new or not previously
released, and (4) found to preferentially attack female gypsy
moth caterpillars or pupae.
References
Fuester, R. 1993. Evaluating biological control potential of
established and exotic parasites. pp. 49-58. In: Fosbroke,
S. and K. Gottschalk, eds. Proceedings, U.S. Department of Agriculture
interagency gypsy moth research forum 1993; 1993 January 19-22;
Annapolis, MD. Gen. Tech. Rep. NE-179. Radnor, PA: Northeastern
Forest Experiment Station, Forest Service; 127 p.
McManus, M., N. Schneeberger, R. Reardon, and G. Mason. 1989.
Gypsy moth USDA Forest Service Forest Insect and Disease Leaflet
162. Radnor, PA: Northeastern Forest Experiment Station, Forest
Service; 13 p.
Reardon, R. 1981. Chapter 6.1 Parasites. pp. 299-421. In:
Doane, C. and M. McManus editors. The Gypsy Moth: Research
Toward Integrated Pest Management. USDA Forest Service Science
and Education Agency Tech. Bull. 1584. Washington, DC.
Reardon, R. and A. Hajek. 1993. Entomophaga maimaiga
in North America: a review. USDA Forest Service NA-TP-15-93.
Radnor, PA: Northeastern Area State and Private Forestry; 22
p.
Reardon, R. and J. Podgwaite. 1992. The gypsy moth nucleopolyhedrosis
virus product. USDA Forest Service. NA-TP-02-92. Radnor, PA:
Northeastern Area State and Private Forestry; 9 p.
Figures
Table 1. Gypsy Moth Parasites Established in the United States
Gypsy moth life stage parasitized
Parasite species
Type of parasite
Imported and Introduced
Egg
Ooencyrtus kuvanae
Wasp
1905-1914
Anastatus disparis
Wasp
1905-1914
Caterpillar
Cotesia melanoscela
Wasp
1905-1914
Phobocampe unicinta
Wasp
1905-1914
Rogas indiscretus
Wasp
1966-1979
Compsilura concinnata
Fly
1905-1914
Parasetigena silvestris
Fly
1922-1933
Blepharipa pratensis
Fly
1905-1914
Exorista larvarum
Fly
1922-1933
Pupa
Brachymeria intermedia
Wasp
1922-1933
Coccygomimus disparis
Wasp
1980-1992



 Back to
Back to